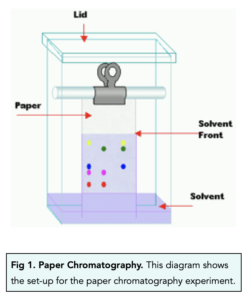
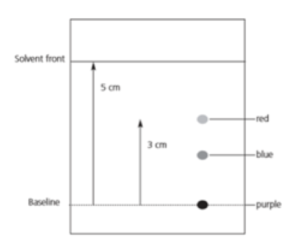
How To Calculate Rf Values
Permit'south get acquainted ?
What is your proper noun?
Nice to meet you lot, {{name}}!
What is your preferred e-mail address?
Nice to meet you lot, {{name}}!
What is your preferred phone number?
What is your preferred telephone number?
Just to check, what are you lot interested in?
When should we call yous?
Information technology would be great to have a 15m chat to discuss a personalised programme and answer any questions
What time works best for y'all? (UK Time)
Pick a time-slot that works best for you ?
How many hours of one-ane tutoring are you looking for?
My WhatsApp number is...
For our safeguarding policy, delight confirm...
Which online course are yous interested in?
What is your query?
Submit
Sure, what is your query?
Submit
Loading...
Thank yous for your response.
We will aim to get back to you within 12-24 hours.
Lock in a 2 Hour 1-i Tutoring Lesson Now
If you're ready and dandy to get started click the button beneath to book your outset 2 hr 1-ane tutoring lesson with usa. Connect with a tutor from a university of your choice in minutes. (Utilise FAST5 to go five% Off!)
Buy At present for £70
How To Calculate Rf Values,
Source: https://studymind.co.uk/notes/chromatography-and-rf-values/
Posted by: hoagmusly1952.blogspot.com




0 Response to "How To Calculate Rf Values"
Post a Comment